Home > Technological Innovation
The all-solid electrolyte that has emerged in recent years is also a way to solve this problem, but due to problems such as low ion conductivity and poor interface contact, the all-solid electrolyte is still in the laboratory stage. Phosphate ester solvents, such as trimethyl triphosphate (TMP), triethyl phosphate (TEP), etc., due to a wider range of operating temperatures, good Li salt solubility, lower viscosity and a wide electrochemical window, The phosphate ester solvent is a good choice for non-combustible electrolytes, but there is a problem with the phosphate ester solvent - it is impossible to form a stable SEI film on the surface of the negative electrode, resulting in delamination and spalling of the graphite negative electrode, and at the same time, the graphite negative electrode surface is catalytic extremely strong, which leads to the continuous decomposition of the phosphate solvent, which also becomes a hurdle for the application of phosphate battery electrolytes.
In order to solve this problem of phosphate electrolytes, Ziqi Zeng (first author) and KeeSung Han (corresponding author) and Ji-Guang Zhang (corresponding author) of Wuhan University proposed a high Li/solvent molar ratio (1 The phosphate ester electrolyte of 2), since most of the solvent molecules in the electrolyte form a solvation structure with Li+, thereby suppressing the occurrence of side reactions of the solvent molecules and the graphite negative electrode, thereby greatly improving the lithium ion battery. Coulomb efficiency (18650 battery coulombic efficiency up to 99.7%) and cycle life.
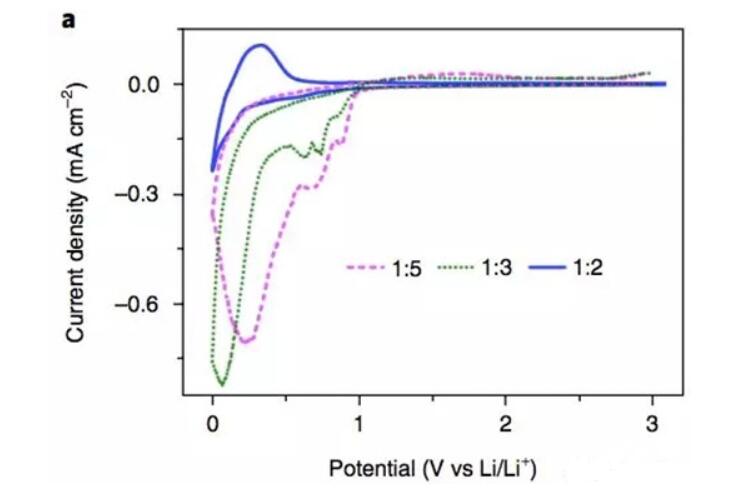
The above figure shows the cyclic voltammetry curve of LiFSI-TEP electrolyte on the surface of graphite anode with different Li/solvent molar ratio. From the test curve, the electrolyte will be in the Li/solvent molar ratio of 1:5 and 1:3. The 0.8V negative electrode undergoes significant decomposition, but when the Li/solvent molar ratio is increased to 1:2, the decomposition current peak of the electrolyte near 0.8V disappears, indicating that increasing the Li/solvent molar ratio can effectively enhance the phosphate solvent. The stability of the graphite surface.
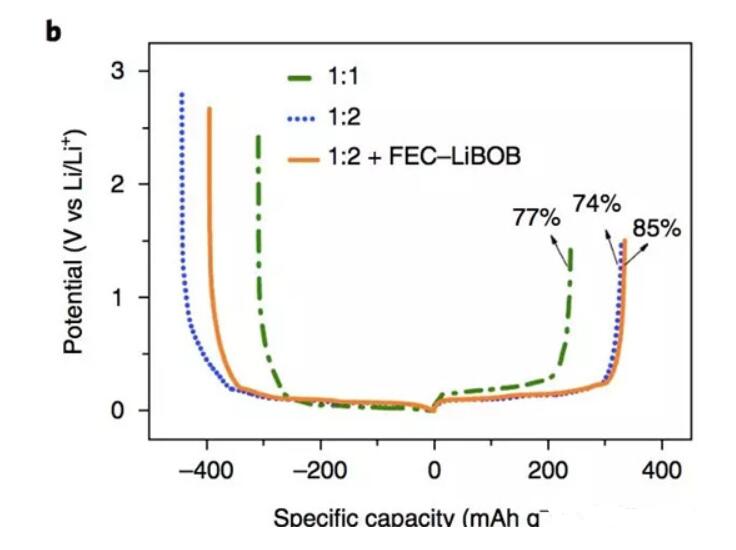
The following figure shows the first charge and discharge efficiency of LiFSI-TEP electrolyte with Li/solvent molar ratio of 1:1 and 1:2 in Li/graphite half-cell, and the first reversible of the two-cell half-cell can be seen. The capacities were 250 mAh/g and 331 mAh/g, respectively, and the first coulombic efficiencies were 77% and 74%, respectively, indicating that the higher Li/solvent molar ratio can well inhibit the decomposition of the solvent on the surface of the graphite anode. The Li/solvent molar ratio is The lower reversible capacity of the 1:1 electrolyte is mainly due to the higher viscosity of the electrolyte and lower conductivity. In order to form a more stable SEI film, the authors also added a small amount of film-forming additives (5% FEC and 0.05 mol/L LiBOB) to the electrolyte, which increased the first efficiency of the graphite negative half-cell to 85%.
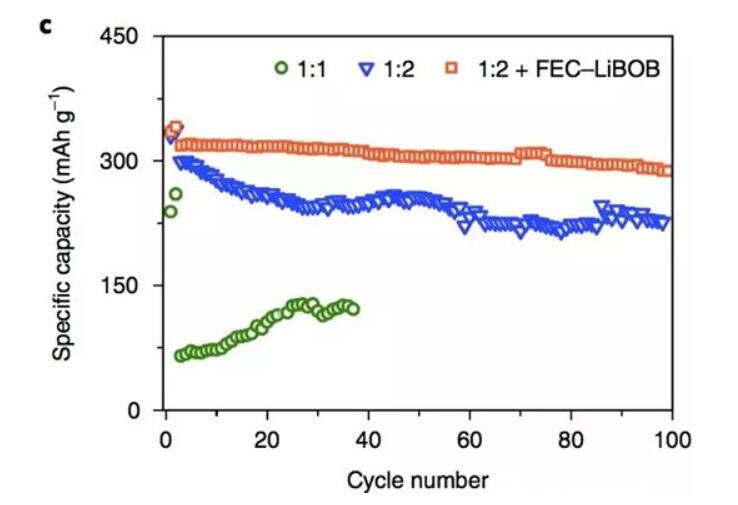
The electrolyte with high Li/solvent molar ratio also showed good cycle performance. From the following figure, it can be seen that the graphite anode has a capacity retention rate of 74% in the electrolyte with a Li/solvent molar ratio of 1:2. Phosphate-based electrolytes with FEC and LiBOB film-forming additives showed more stable cycle performance, with a capacity retention rate of 91% in 100 cycles and a coulombic efficiency of 99.8% in the cycle. It is almost comparable to carbonate electrolytes. The mechanism study found that the SEI film formed in the electrolyte containing FEC and LiBOB has higher LiF content (FEC decomposition product) and organic oligomer content (LiBOB decomposition), and this dense organic-inorganic composite SEI film. It can better reduce the contact between TEP and graphite, thereby reducing the decomposition of phosphate solvent and improving the cycle life and coulombic efficiency of the negative electrode.
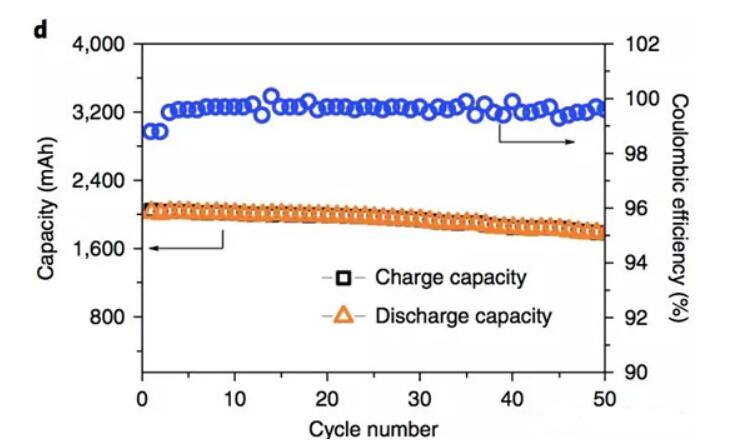
In order to verify the performance of the above non-flammable electrolyte in the whole battery, ZiqiZeng tested the phosphate ester electrolyte with FEC and LiBOB film-forming additives using LCO positive electrode and graphite negative electrode 18650 battery. The figure below shows the battery at 0.05C. The data of the cycle under the magnification can be seen from the figure. The capacity retention rate of the whole battery is 90% after 50 cycles (the battery capacity retention rate of the carbonate electrolyte is about 94%), and the charge and discharge coulombic efficiency reaches 99.7. %.
Although there is still a gap between the phosphate non-combustible electrolyte and the commercial carbonate electrolyte in terms of cycle performance, the use of this non-combustible electrolyte greatly improves the safety performance of the lithium-ion battery. In the acupuncture and extrusion tests, the 18650 battery with non-combustible electrolyte passed all tests, while the battery with commercial carbonate electrolyte only passed the extrusion test, especially the battery with non-combustible electrolyte in the needling test. The integrity of the structure was maintained (the middle cell in the figure below), and no combustion occurred, and the battery using the commercial carbonate electrolyte occurred in the acupuncture test, and the entire battery was burned (lower battery in the figure below).

By using a high Li/solvent molar ratio, Ziqi Zeng increases the solvation ratio of solvent molecules, thereby greatly improving the stability of the phosphate electrolyte on the surface of the graphite negative electrode, improving the charge-discharge coulombic efficiency and cycle performance, Phosphate electrolytes have comparable performance to carbonate electrolytes, and the non-combustible properties of phosphate electrolytes greatly enhance the safety properties of lithium-ion batteries, helping lithium-ion batteries pass through extrusion, acupuncture and Short circuit test.
AOT ELECTRONIC TECHNOLOGY CO.,LTD which has 10 years experience in LITHIUM ION BATTERY field. We provide full kinds of battery equipment and material, the lab research line is available according to the requirements of customer.
Advantage of Technology, Advantage of Team!
Contact: Ms.Lika (Sales)
Email/Skype: sales@aotbattery.com
Phone: 0086-18250729722
Web: www.aotbattery.com
Contact: Lika
Phone: +86-19906035385
Tel: 0086-592-7161550
Email: sales@aotbattery.com
Add: No.168, Zhaogang Road, Xiamen City, China